MODEL EXPLORATION
Home » Solutions / Preclinical EEG Services / Preclinical Assays / Model Exploration
The Challenge of Translatability
In preclinical development, an important question is whether the models used can shed light on the mechanisms leading to the manifestations of neurological diseases, validate drug targets, and provide the necessary confidence that a compound or therapeutic approach will ultimately benefit patients.
A major hurdle in the development of therapeutics is the issue of translatability from rodent models to humans. It is notably difficult to fully model a disorder even for single-gene disorders. Moreover, promising results in rodent models for many therapeutics fail to translate to therapeutic value for human patients in clinical trials.
To overcome this challenge, biomarkers are needed to link patients and animal models, to boost translatability, and to help you get the best out of your chosen model.
Preclinical EEG: Boost Your Drug Discovery with Translational EEG Biomarkers for Your Models
Electroencephalography (EEG) is one of the few techniques measuring neural activity that can be recorded in rodents and humans alike while the subject is resting or performing sensory or cognitive tasks. This powerful technology allows us to carry out reverse translational studies by replicating observations from humans in rodents.
SynapCell’s Model Exploration solution offers to search for a translational, objective EEG biomarker in freely-moving animals, in the model of your choice.
Translational Technology
At the preclinical stage, EEG monitoring and evoked potentials in freely-moving animals:
- Are implemented in the same conditions as in humans
- Help improve your model’s predictability and reliability

Expertise
A third of our team are PhD-holders, with the capacity to counsel so you can make the most of your model. Together, we will craft a study tailored to your needs.
Sensitivity & Precision
EEG biomarkers can discriminate between males & females, between genotypes (heterozygotes / homozygotes), and even between adult versus aged rodents.
3 Steps to Phenotyping a Model and Identifying an EEG Biomarker
Step #1
Identify the EEG Signature
EEG SIGNATURE IDENTIFICATION
Identify or confirm a phenotype.
Step #2
Validate as an EEG Biomarker
VALIDATION AS AN EEG BIOMARKER
Reversible with reference compounds, convert aberrant EEG oscillations into a relevant biomarker.
Step #3
Test Compound
COMPOUND TESTING
Determine the efficacy levels of various compounds.
As our Cue® platform is patient-inspired, we can carry out reverse translational studies. Initially looking for an EEG biomarker in the clinic, then attempting to replicate it in your model.
This paradigm can also be deployed for personalized medicine, by looking at how a mutated gene affects brain activity.
POSTER
Characterization of a Transgenic Mouse Model of Tauopathy Using Quantitative EEG: Does Tau Pathology Lead to Disrupted EEG Spectra?
We took advantage of quantitative electroencephalography (qEEG) to characterize a transgenic mouse model of tauopathy. We implanted female P301L and wild-type (C57BL6) mice with stainless steel screws over the frontal and parietal cortices, as well as a reference electrode placed above the cerebellum. After acclimatization for 1 hour in our recording chambers, EEG was recorded continuously for 120 min.
DOWNLOAD
POSTER
DOWNLOAD
POSTER
POSTER
EEG Phenotyping as a Loop Translation Tool to Address the Pathophysiology of GABA-A Signaling-associated Brain Disorders
EEG endpoints are commonly used in the clinic to identify and evaluate a pathological state and/or as surrogate biomarkers for clinical trials. In this work, we took advantage of quantitative electroencephalography (qEEG) to characterize a humanized transgenic mouse line carrying a mutation in the GABA-A signaling, a mutation observed in patients with rare diseases.
“I am very pleased with our collaboration with SynapCell investigating EEG phenotypes in a Tauopathy mouse. The work at SynapCell was very high quality, the results were delivered on time, and the people were a pleasure to work with.”
Frank Menniti, CSO of MindImmune Therapeutics

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s rodent models and their associated EEG biomarkers are processed on on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
What Would You Like to Assess in Your Model?
EPILEPTIC ACTIVITY
EPILEPTIC ACTIVITY
Identification of spontaneous seizure activity.
Abnormal Brain Activity
Abnormal Brain Activity
Identification of alterations compared to control in specific frequency bands by quantitative EEG (qEEG). Disturbance of Phase Amplitude Coupling.
Quality of information processing by the model
Quality of information processing by the model
Modification of 40Hz ASSR / AERPs (power, inter-trial coherence).
EEG is sensitive enough to quantify the phenotype of a model based on the number of copies of a gene. This opens the possibility of using models for personalized medicine, characterizing a mechanism of action, or validating a drug target.
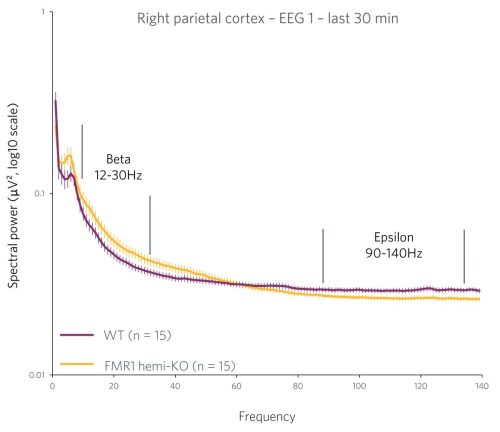
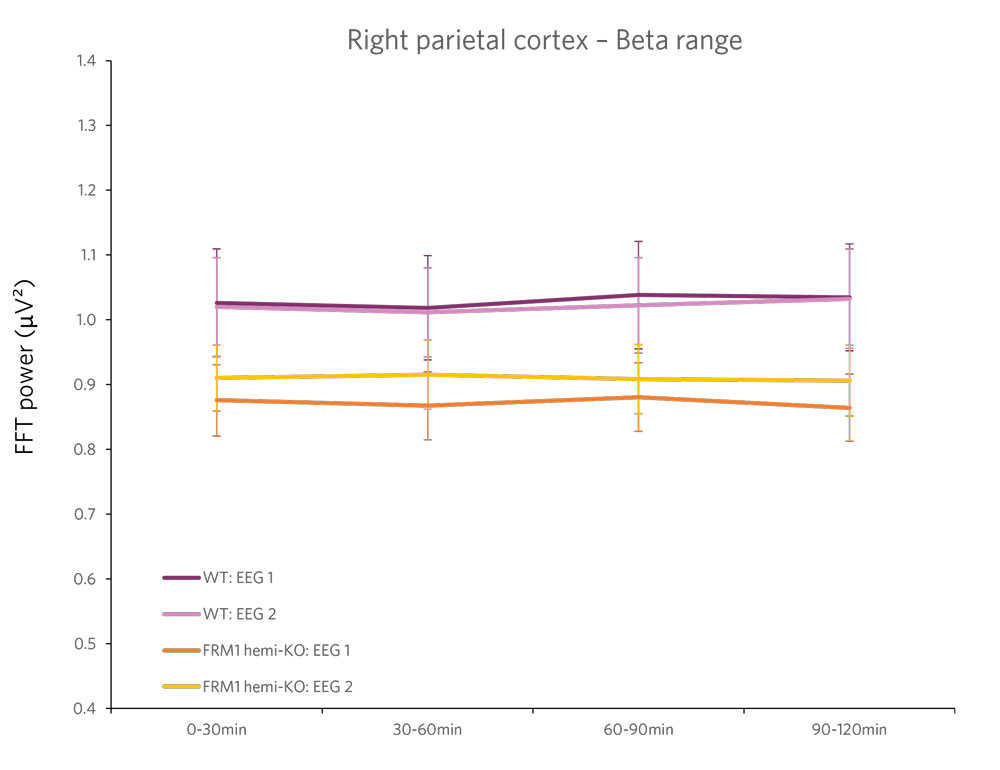
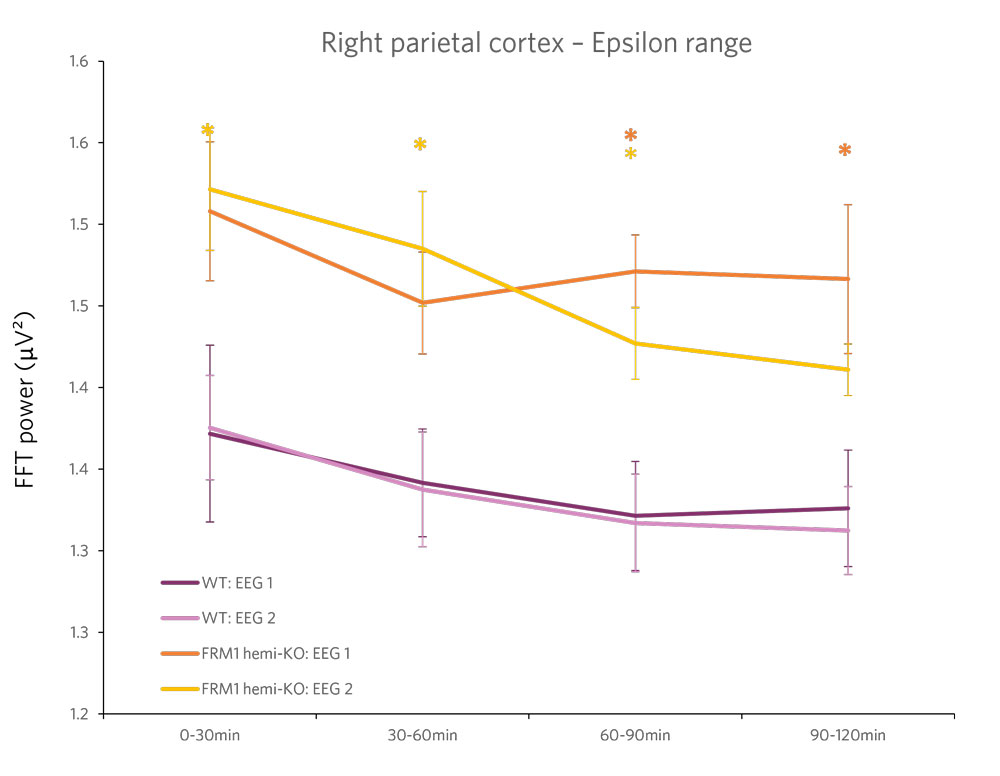
Case Study: Phenotyping a Model of Fragile X Syndrome
Figure 2 : Quantification of the beta frequency band (left) (between 12 and 30 Hz) and of the epsilon frequency band (right) (between 90 and 140 Hz). A power decrease is observed in the Beta range, and a significant increase in power is observed in the Epsilon range for FMR1-KO rats compared to WT rats.
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we’re your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.