AutisM Spectrum
Disorder
Home » Solutions / Drug Efficacy Testing / Rare Disorders / Autism
The Growing Burden of Fragile X Syndrome,
Affecting Autism Spectrum Disorders
Fragile X Syndrome (FXS) is recognized as the most common cause of inherited intellectual disability as well as the most commonly known cause of autism spectrum disorders (ASD). FXS affects approximately 1 in 3,600 males and 1 in 4,000-6,000 females(1). It is therefore one of the most prevalent rare neurological conditions worldwide (Orphanet).
FXS is a genetic condition caused by a lengthening of the Fragile X messenger Ribonucleoprotein (FMR1) gene on the X chromosome.
Considering these facts, Fragile X Syndrome, as several other types of rare CNS conditions, is being increasingly investigated by drug developers. Although relatively efficient therapeutic strategies have been developed, their efficacy is insufficient. The CNS community needs to fill this gap. Electroencephalography could be a game changer, helping the scientific community thanks to its well-known ability to bridge the preclinical to clinical gap.
The FMR1-KO rat, a Translational Model of Fragile X Syndrome
Patients with FXS show a significant decrease in the evoked response to auditory stimulations. Resting state qEEG also appears to be a relevant second endpoint, with patients showing an increase in high frequencies (between 40 and 120Hz) and a decrease in low frequency bands (between 10 and 20Hz).
Building on our years of research and development efforts, we characterized a translational model of Fragile X Syndrome, the FMR1-KO rat. This model closely mimics the evoked responses from auditory stimuli reported in the clinic.
*Erthridge et al, 2017
The FMR1-KO Rat, a Reference Model in the Field of ASD
The FMR1-KO rat model shows deficits in information processing, increased baseline gamma power, and decreased alpha power. This profile reflects clinical (in-patient) observations, with reduced local circuit inhibition leading to sensory hypersensitivity and neuro-excitability.
A Robust and Translational EEG Biomarker
ASSR abnormalities are associated with conditions like Autism Spectrum Disorders (ASD).
Thanks to the highly translational properties of EEG, coupled with our proprietary analysis platform Cue®, we have identified a relevant EEG biomarker, the 40Hz-ASSR. This EEG biomarker is found in humans and rodents, and evaluates auditory cortex function and neural synchronization.

Paving the Way for New Predictive Models
In addition to providing efficacy models, SynapCell can also help to identify abnormal brain wave patterns in animal models using EEG phenotyping. By focusing on Auditory Evoked Potential (AEP) protocols, we have developed a predictive approach, expanding our programs to test efficacy during drug discovery.
Key Features
of the FMR1-KO Rat Model
The FMR1-KO rat model is considered a gold standard for drug discovery in FXS disease:
- Gamma synchronization of ITC and ERP reduction in FRMR1-KO rats
- Information processing deficits are identified in FMR1-KO rats
- Epsilon power increases significantly after qEEG analysis
- GABAergic inhibitory neurons are involved in rats
Cue®, SynapCell’s platform, is a reliable solution to evaluate potential treatments for Autism in preclinical research.
Correlation between gamma power and sensory hypersensitivities in FXS patients converge with findings from animal models of FXS, with their altered local inhibitory networks. The model is thus relevant to help you identify the most promising compounds in terms of their functional ability to reverse deficits.
Drug Discovery Assays
with the FMR1-KO Rat Model
The stability of the model and its endpoints over time make it possible to perform different types of experimental protocols. In acute conditions, each animal can be used as their own control as part of a crossover protocol, enhancing the power of statistical analyses.
In summary, this model is suitable for identifying, optimizing and testing promising compounds in terms of efficacy and pharmacological properties to determine their potential as drug candidates.
Assess how a compound’s effect changes with varying doses and analyze the relationship between dose and magnitude of the response, dose and effect duration; examine efficacy, safety, and biological effects with a range of concentrations to determine optimal dosing strategies.
POSTER
Alteration in Spontaneous and Auditory Evoked Electro-physiological Activities in a Rat Model of Fragile X Syndrome
In this study, we see that the sensitivity of EEG allows a dynamic characterization and differentiation of translational humanized models of brain disorders. The combination of translational preclinical models with EEG represents the next step for feedback from preclinical trials into clinical practice, paving the way for precision medicine for patients.
DOWNLOAD
POSTER
AUDITORY EVOKED
POTENTIAL
APPLICATION NOTE
40Hz-Auditory Steady State Response (40Hz-ASSR)
The main challenge in preclinical research is identifying biomarkers common to clinical and preclinical conditions. The 40Hz-ASSR is a non-invasive EEG biomarker that is detectable in both humans and rodents. It measures how well the auditory cortex processes information and generates synchronous activity at a specific frequency. Disruptions to the ASSR are linked to disorders such as autism spectrum disorder.

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s FMR1-KO rat model and its associated 40Hz-ASSR EEG biomarker are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
Insight into the 40Hz ASSR Paradigm
The 40Hz ASSR is of interest because it reflects synchronous neural oscillations in the auditory cortex and is thought to be associated with cognitive processes like attentiveness and sensory integration. The frequency range is significant in neuroscience and clinical settings, where abnormalities in 40Hz ASSR have been linked to various neurological and psychiatric conditions, including schizophrenia and autism.
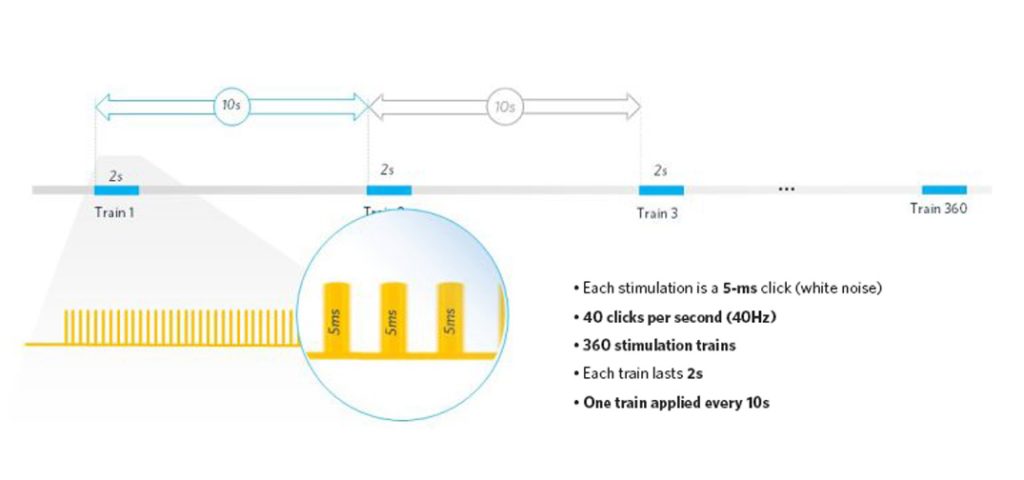
SynapCell uses a specific paradigm in-house, delivering a train of 80 auditory stimulations within two seconds, repeated 360 times. Each auditory stimulation is a 5-ms white noise (click). By measuring the inter-trial coherence (ITC) between the 360 repeats, we can collect information on how the brain filters redundant information and whether it adapts over time.
What sort of Translational Readouts are Provided by our Experts?
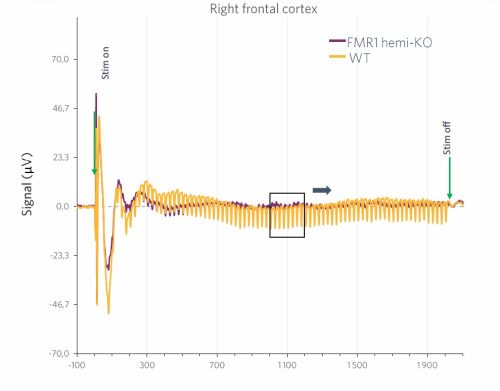
Results are presented as comparisons of the evoked responses to the 40Hz ASSR paradigm for wild-type animals (WT) and FMR1-hemi-KO rats. In this sample graph, we can clearly observe a reduction in the average response in FMR1-KO rats compared to WT animals.
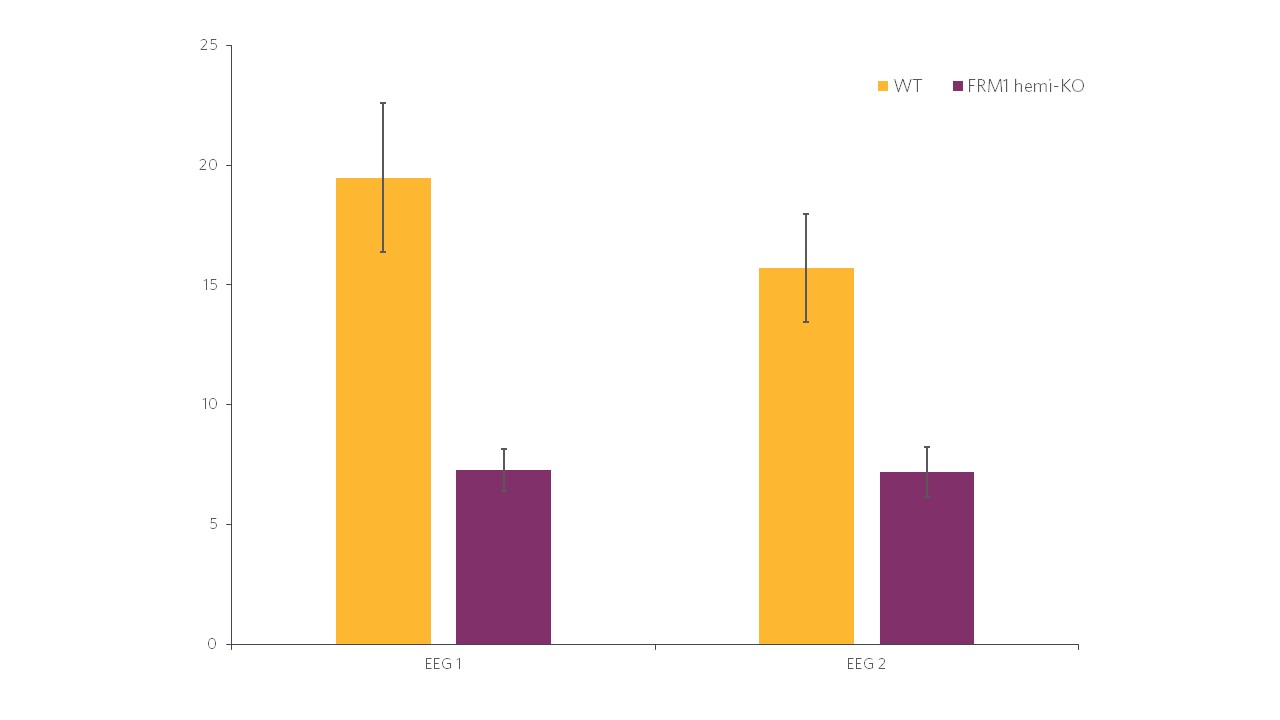
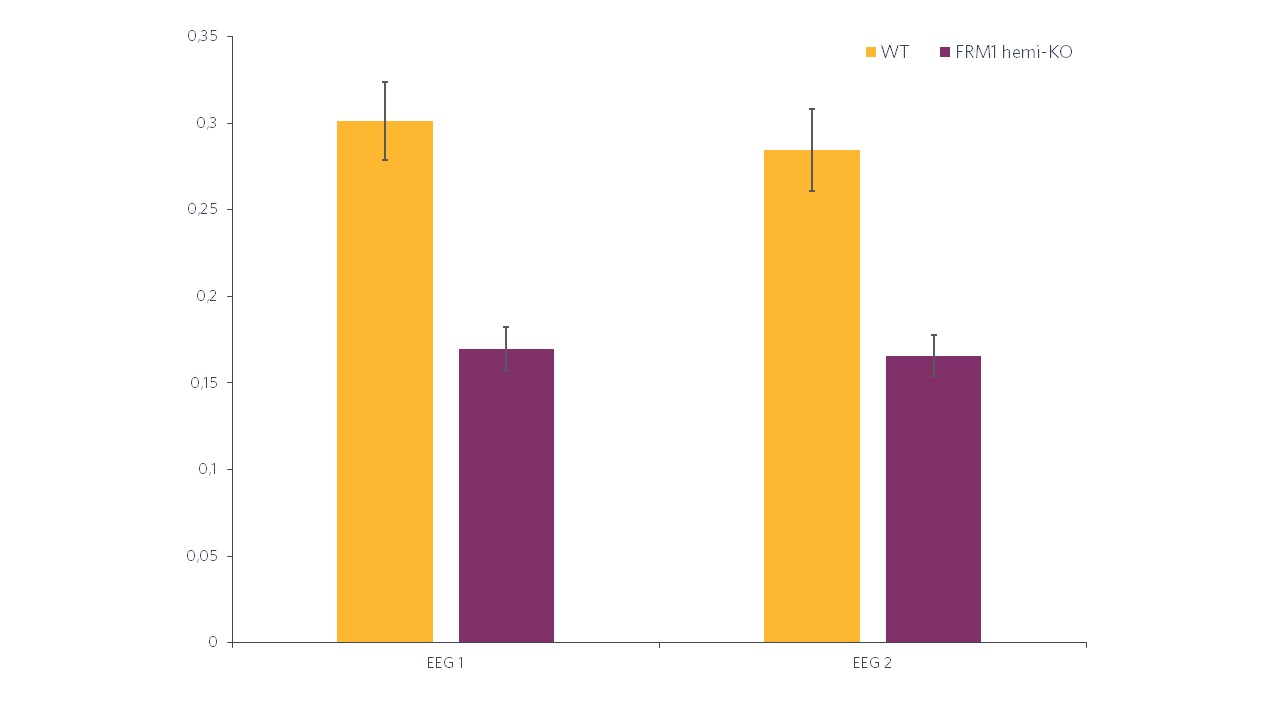
Histogram representations (Figure 2) reveal a significant decrease in the evoked power in FMR1-KO rats compared to WT rats. In terms of the ITC, in Figure 3 we can also see a significant decrease in FMR1-KO rats. These observations suggest that FMR1-KO rats have a deficit in information processing compared to their WT littermates.
The sensitivity of EEG allows dynamic characterization and differentiation of translational humanized models of brain disorders. By combining translational preclinical models with EEG, we have already taken the next step to feedback from preclinical trials to influence clinical practice, paving the way for precision medicine for patients.
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.